21/02/2019
Print PageColon cancer: Different Wnt signaling pathways lead colon cells astray
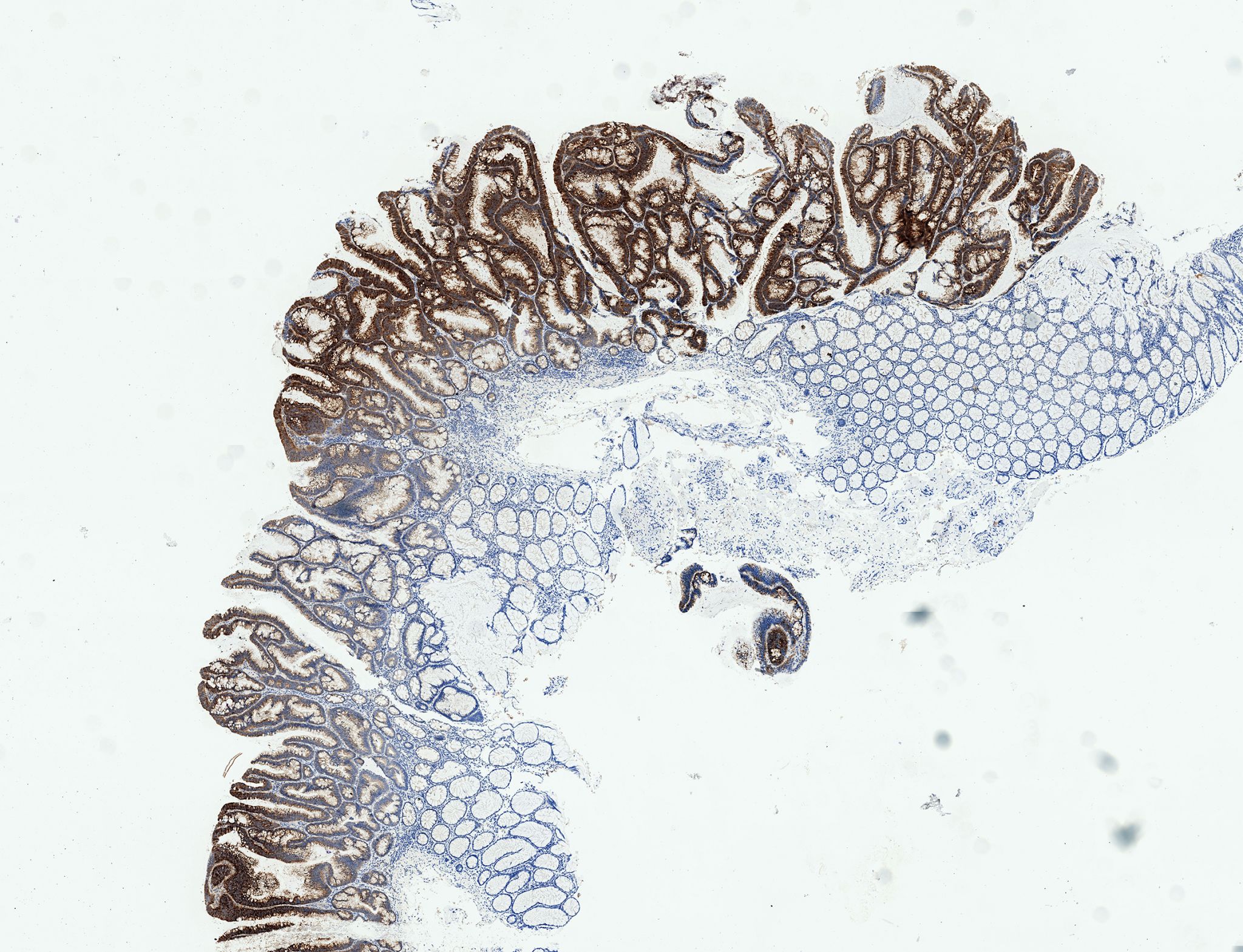
Around 80 percent of all colon tumors involve mutations of the tumor suppressor protein APC (Adenomatous polyposis coli), which leads to an overactive Wnt signaling pathway. Wnt is therefore regarded as one of the most important biomarkers for colon cancer and as a possible target for new colon cancer therapies. “Previous attempts to block the Wnt signaling pathway have failed, however, because of the strong side effects,” explains Henner Farin, head of a DKTK young investigator group at Georg Speyer House in Frankfurt. “This is because the Wnt messenger is also essential for stem cell formation and cell division in healthy cells.”
In this study, Henner Farin and his colleagues discovered for the first time a tumor-specific molecular reaction chain that is triggered by Wnt. The researchers used three-dimensional colon cultures (‘organoids’) that they had grown from healthy human colon tissue. Organoids represent a new method in biomedical science. Intestinal cells are reproduced as a united cell structure in the laboratory so that they incorporate features similar to the organ they represent and, for example, can be studied to investigate how drugs work in the colon. Using these mini-guts, the researchers were able to compare Wnt activity in colon tumors and in the stem cells from a healthy gut. To do this, they switched off the tumor suppressor protein APC, which caused tumor precursor cells (adenomas) to form. Interestingly, this caused Wnt to activate a completely different palette of genes than in the healthy cells.
“Here we see two completely different molecular responses between the healthy cells and the tumor cells,” says Henner Farin. “This means that the increased Wnt signal per se is not significant for diagnosis and prognosis. You have to look at the downstream gene expression patterns.” The results also explain why until now there have been contradictory reports: sometimes a high Wnt signal is linked to a poor prognosis and sometimes to a good one.
The researchers correlated the different expression patterns with the molecular data of large patient cohorts and were then able to identify subtypes with favorable and less favorable disease progression. In addition, the team identified a range of protein markers that could be used to detect tumors in the future.
The researchers see great potential in their organoid approach for discovering and targeting new vulnerabilities in tumor cells without harming the healthy cells. “Until now, there were no suitable in vitro models for neatly comparing healthy cells and tumor cells,” says Henner Farin. “Patient samples are genetically very variable and there is often no comparison with normal cells. With organoids, we can zoom in on the tumor-specific components in cellular signaling pathways for other types of cancer as well, and discover new cancer-specific vulnerabilities.”
Original publication:
B. E. Michels et al. Human colon organoids reveal distinct physiologic and oncogenic Wnt responses. In: Journal of Experimental Medicine. (Online publication 21 February 2019) DOI 10.1084/jem.20180823