05/06/2019
Print PageStomach and colorectal cancer: Identifying patients at an early stage who are suitable for artificial intelligence immunotherapy.
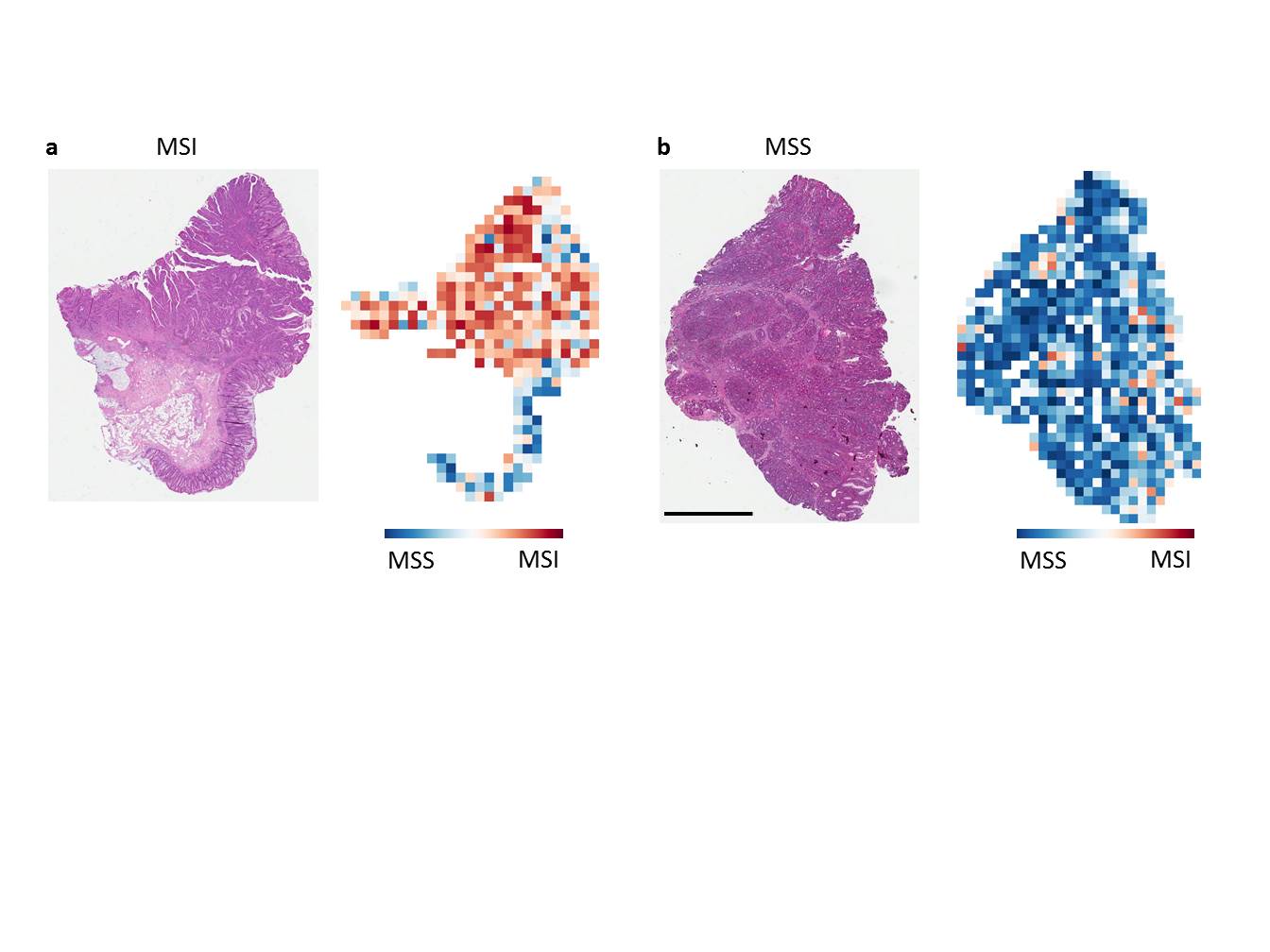
Only a small number of patients with stomach or colorectal cancer respond to immunotherapy. Some tumors lead to changes in the genetic material, and this can cause mutations in the sections of the genome, referred to as “microsatellites”, that are frequently replicated. This microsatellite instability (MSI) is a characteristic for distinguishing between different cancers of the gastrointestinal tract and determines whether patients with these diseases are able to respond well to immunotherapy with checkpoint inhibitors. Detecting these properties usually requires a genetic or immunohistochemical test, which requires additional costs and is not always automatically performed for every patient in clinical practice.
The scientists in Aachen and Heidelberg, in collaboration with international colleagues, showed that a computer-aided adaptable algorithm, based on the concept of “deep learning”, enables MSI to be directly diagnosed based on routinely available images of tissue samples without the need for additional laboratory tests. “With our approach, we have the potential to test any patient with colorectal cancer for MSI automatically and cost-effectively, which allows us to provide the option of immunotherapy to a larger group of colorectal cancer patients,” says Jakob Nikolas Kather, physician and scientist at the Clinic for Gastroenterology, Metabolic Diseases and Internal Intensive Care (Medical Clinic III) at Uniklinik RWTH Aachen and member of staff at DKFZ and NCT Heidelberg. “This makes it possible to identify patients who may otherwise never be considered for immunotherapy. However, this approach must be reviewed in prospective studies,” adds Dirk Jäger, Medical and Executive Director of the Department of Medical Oncology at NCT Heidelberg.
Immunotherapy for cancer
Cancer immunotherapies with so-called checkpoint inhibitors, which can be seen as active agents that release the “brakes” of the immune system, have attracted considerable attention in recent years. For colorectal cancer, however, checkpoint inhibitors have thus far only been successful in “microsatellite instable” tumors. In the more common “microsatellite stable” cases of colorectal cancer, checkpoint inhibitors have not shown objective response rates in previous studies. It remains challenging to predict which patients can benefit from immunotherapy in daily practice. This makes it all the more important to identify patients at an early stage who could benefit from immunotherapy in day-to-day clinical treatment.
Artificial intelligence in medicine
Artificial intelligence and adaptable algorithms are becoming increasingly prominent in medicine. Technological support has been required for a long time. The relevant data are entered into the applications, thereby training the algorithm to analyze large amounts of data and medical images to enable more efficient and more accurate treatments, such as the automatic detection of cancer cells.
Original publication
Jakob Nikolas Kather, Alexander T. Pearson, Niels Halama, Dirk Jäger, Jeremias Krause, Sven H. Loosen, Alexander Marx, Peter Boor, Frank Tacke, Ulf Peter Neumann, Heike I. Grabsch, Takaki Yoshikawa, Hermann Brenner, Jenny Chang-Claude, Michael Hoffmeister, Christian Trautwein, Tom Luedde (2019) Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nature Medicine, https://www.nature.com/articles/s41591-019-0462-y